Three main research branches
My research group at the Medical University of Vienna (MedUniWien) focuses on three main topics:
- BrainBuild: Human fetal brain malformations
- BrainPRC2: The role of Polycomb in brain development
- RareTumors: modeling rare pediatric brain tumors in vitro using patient-derived iPSCs from individuals presenting with tumor predisposition syndromes
Our current funding:



Team
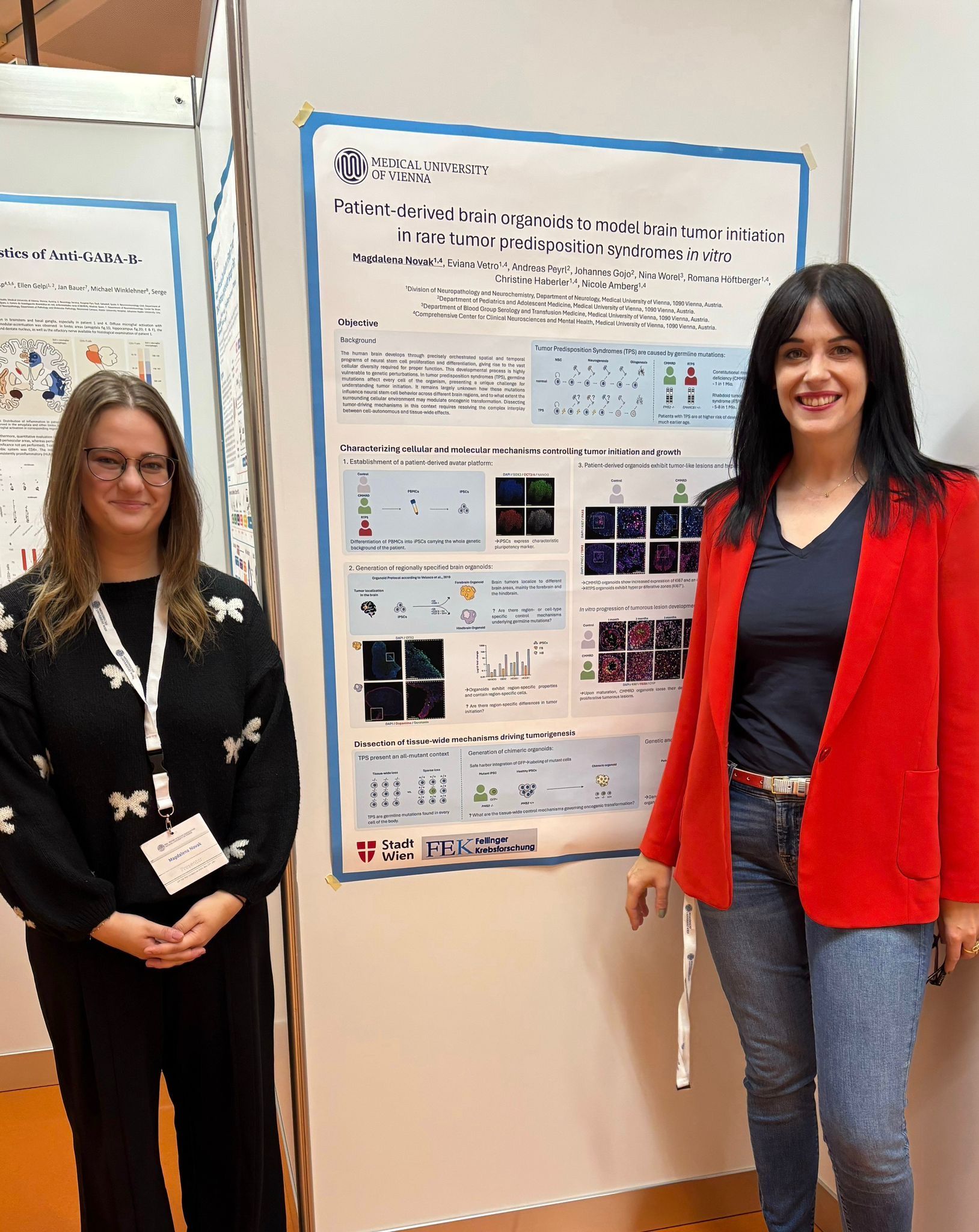
Magdalena Novak, BSc
Magdalena is a highly talented Master's student in the Master program Neuroscience at the University of Vienna. She is performing reprogramming of patient-derived PBMCs and establishes regionally specified brain organoids (forebrain and hindbrain) from our patient-derived iPSCs.
She has been able to show that patient-derived forebrain organoids develop hyperproliferative zones and tumor-like lesions, while at the same time displaying a dysintegration of rosettes. She will assess whether brain organoids with distinct regional patterning show different dynamics in tumor initiation. Furthermore, she will perform molecular profiling of patient-derived organoids and compare the results to profiles obtained from patient tumor biopsies.
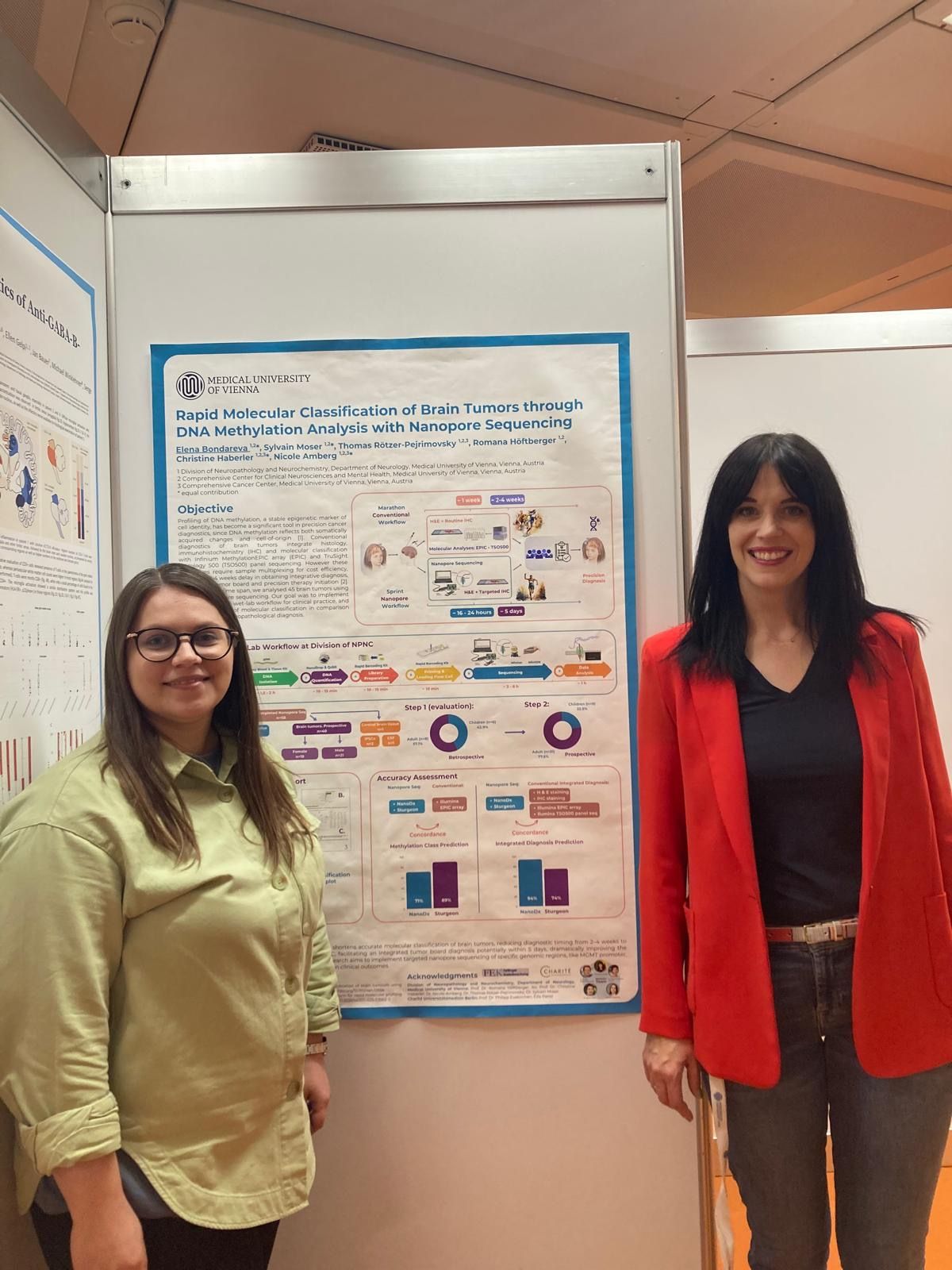
Dr. Elena Bondareva
Elena is a trained neurologist and currently obtaining her Master's degree in Molecular Precision Medicine at the Medical University of Vienna. With rigor and precision, she has established an applicable workflow for rapid methylation-based brain tumor classification using nanopore sequencing.
Elena's work has brought cutting-edge rapid molecular diagnostics to application in our Division. In a streamlined workflow using random genome-wide sequencing on a MinION device, she has implemented brain tumor classification in 8-24h (depending on the reusage performance of the flow cell). In a next step, Elena aims to achieve intraoperative ultra-fast classification, obtaining results within 90min. Additionally, she will establish adaptive sampling to specifically screen therapy-relevant loci, such as the MGMT promoter or the BRAF gene.
Alumna: Eviana Vetro (erasmus+ student)
Eviana is a Master's student from the University of Cologne, who performed pluripotency validation in one of our patient-derived iPSC lines. She could also show comparable behavior of healthy donor-derived iPSCs and patient-derived iPSCs in terms of proliferation rate, expression of pluripotency markers and ability to give rise to cerebral organoids.

Alumnus: Maximilian Hanner (FFG intern)
Maximilan attends Med School at the Medical University of Vienna. He has performed histological stainings for H3K27me3 on human fetal cerebellar tissue, where he observed dynamic, cell type-specific changes in H3K27me3 levels in the course of cerebellar development. He also became interested in a rare cerebellar malformation called rhomboencephalosynapsis, which shows distinct H3K27me3 levels.

BrainBuild: Mechanisms of human fetal brain development
The characterization of distinct cell types in human fetal brain malformations is surprisingly superficial. In order to overcome this knowledge gap, I am making use of my knowledge on particular cell type markers to visualize the various progenitor, neuron, and glia populations in fetal tissue archived in the impressive neurobiobank at the Division of Neuropathology and Neurochemistry at MedUniWien.
The resulting detailed description of cellular alterations in distinct malformations, such as hemimegalencephaly, will be highly valuable to the community as important reference for experimental models besides awareness about our biobank for research purposes.
Happy to collaborate!
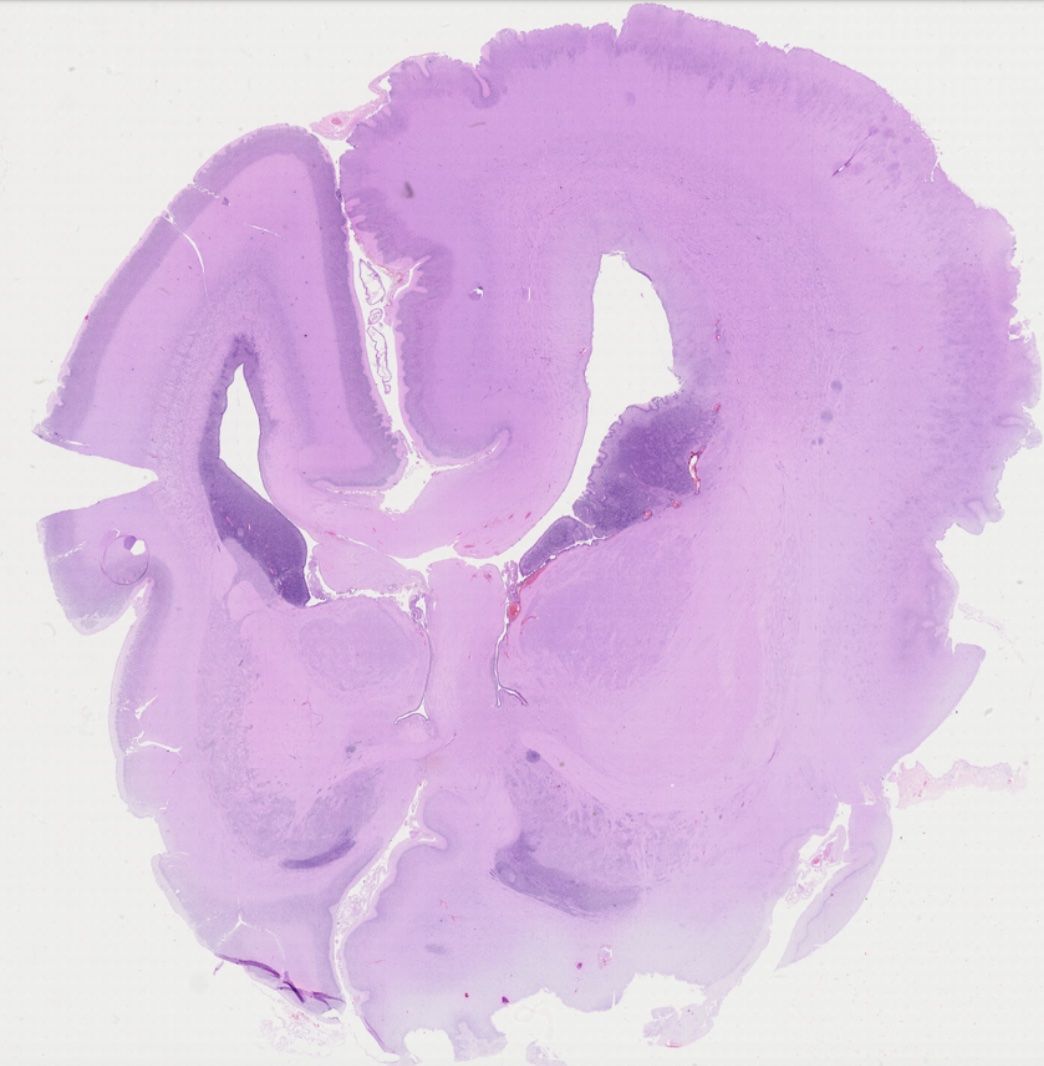
GW22 hemimegalencephaly
BrainPRC2: Understanding PRC2 function in human brain development
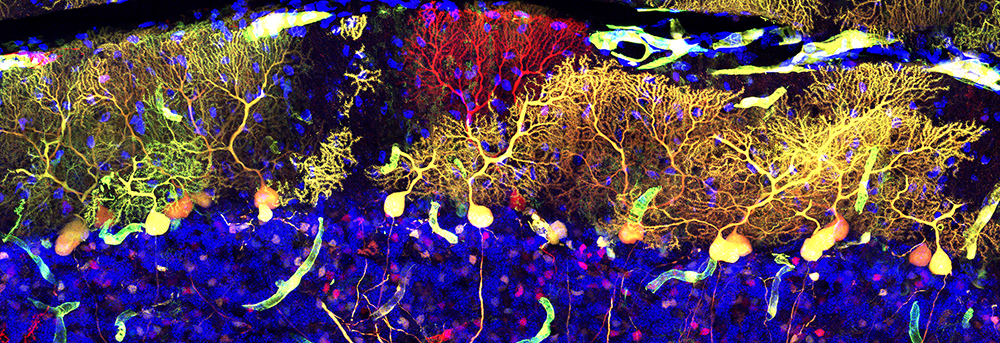
In order to assess the function of the epigenetic regulator Polycomb Repressive Complex (PRC)2 in human brain development, we are applying immunohistochemistry stainings for H3K27me3 to human fetal brain FFPE sections. This approach allows us to monitor PRC2 dynamics across developmental stages and cell types in the human brain. Starting in the cerebellum, we observe H3K27me3 loss in the IGL over time, correlating with granule cell development. In contrast, we detect retainment of high H3K27me3 levels in the Purkinje cell layer.
In a rare cerebrallar malformation termed rhomboencephalosynapsis, where the vermis is completely missing and the cerebellar hemispheres are fused, we see higher H3K27me3 levels in the IGL as compared to age-matched controls. This rather immature state correlates with an immature appearence of Purkinje cells, potentially indicating an overall immature state of the cerebellum in the malformed condition.
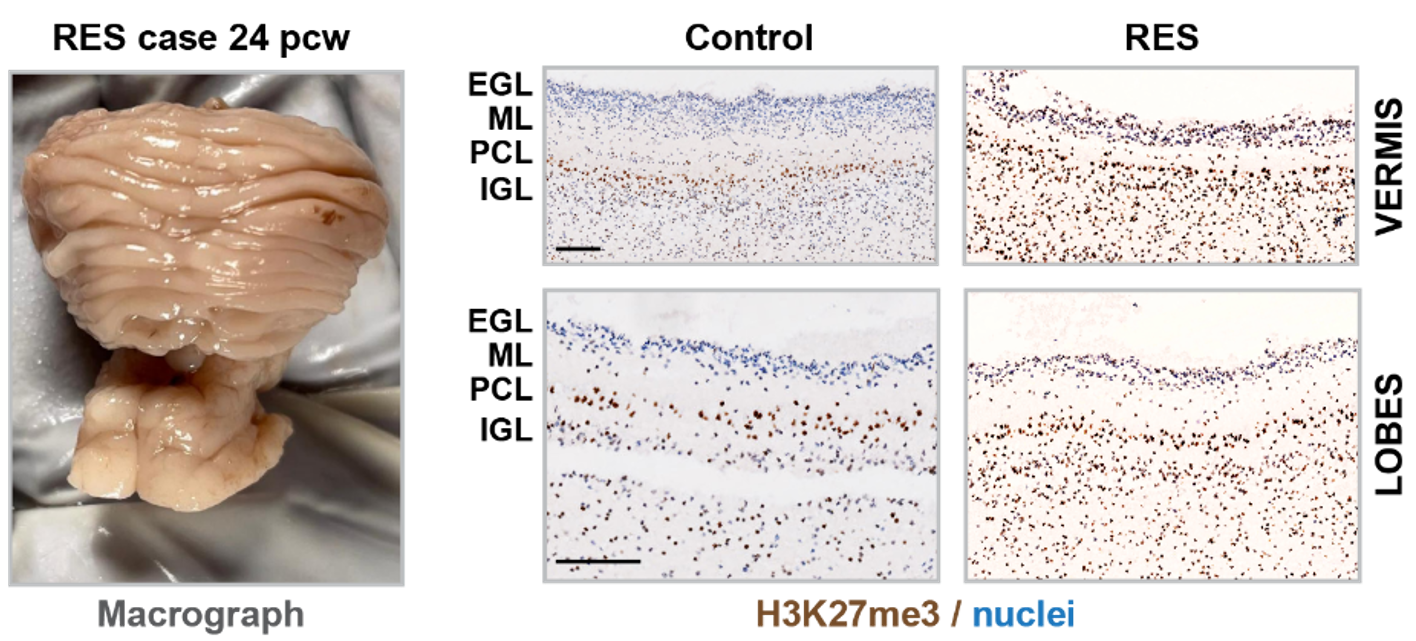
Human fetal cerebellum: comparison of H3K27me3 levels in control and rhomboencephalosynapsis
RareTumors: Understanding the mechanisms of pediatric brain tumor development
This part of my research focuses on dissecting the cell-autonomous and non-cell-autonomous mechanisms of pediatric brain tumor development in patients suffering from tumor predisposition syndromes. These syndromes are caused by germline mutations and account for 8-19% of pediatric brain tumors, of which some show highly aggressive behavior and are thus associated with a poor prognosis. In contrast to spontaneous somatic mutations, germline mutations provide a particularly challenging setting for the precise assessment of tumor-driving mechanisms based on an all-mutant cellular context of the organism.
Being positioned in the clinical setting of the Division of Neuropathology and Neurochemistry at the Medical University of Vienna grants me direct access to precious patient material and provides me with excellent state-of-the-art expertise in neuropathology and molecular diagnostics. Thus, we are generating iPSCs from patients presenting with tumor predisposition syndromes and cultivate brain organoids to mimic tumor initiation in vitro.
This branch of our research aims to answer the following open questions:
- What is the precise identity/state of a mutant neural stem cell when undergoing oncogenic transformation?
- What is the sensitive developmental time window for oncogenic transformation?
- How are the above mentioned parameters (individual stem cell behavior, mutant stem cell state and tumor initiation) shaped by cell-autonomous (i.e. cell intrinsic) and non-cell-autonomous (i.e. systemic or tissue-wide) cues elicited by the mutant cell(s)?
- What are the divergent pathways among particular tumor predisposition conferring tumor-specific features such as aggressiveness?
Here is a glimpse into our first patient-derived iPSC lines and cerebral organoids:
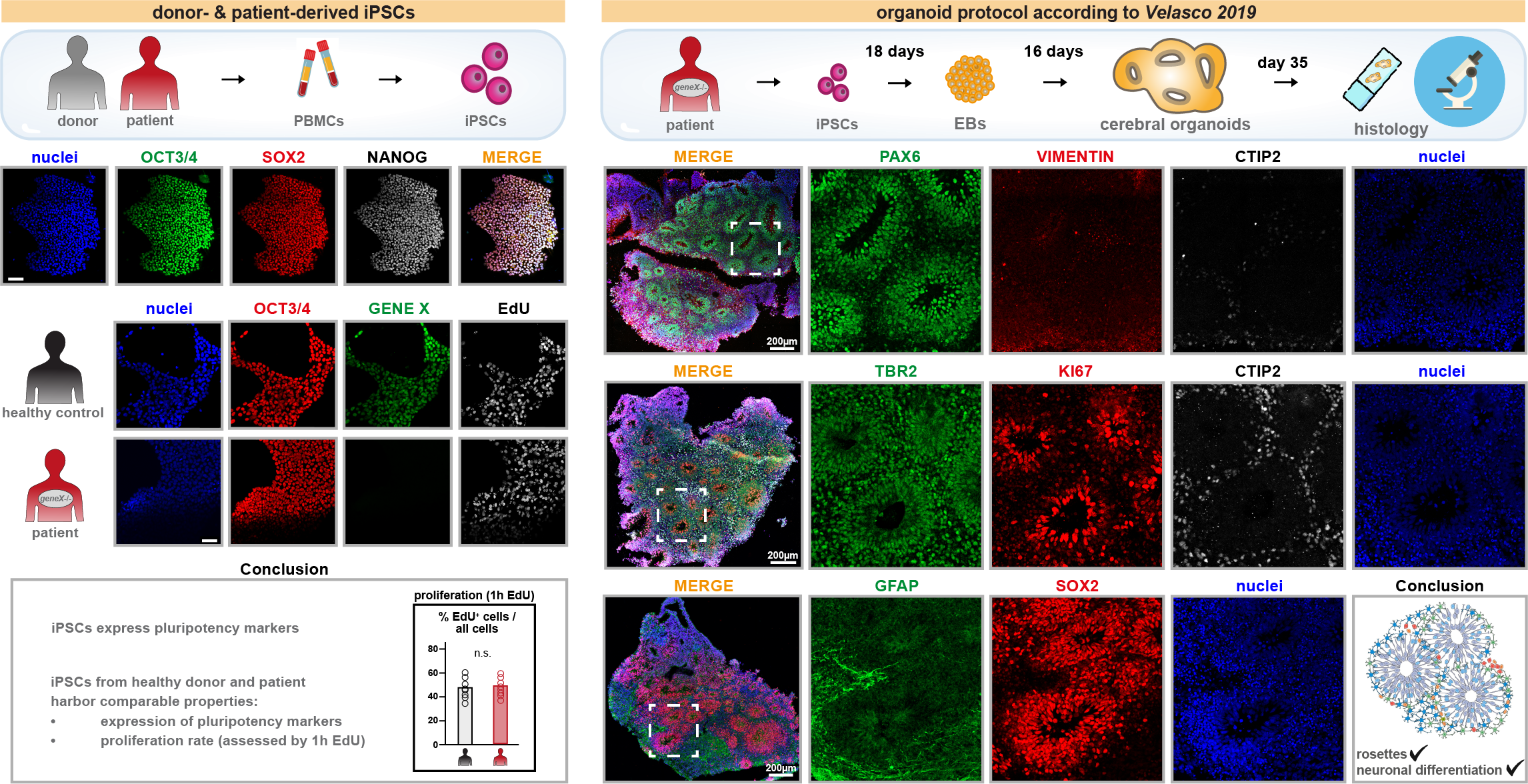
Foundation:
My postdoctoral work uncovering distinct and sequential functions of the epigenetic regulator Polycomb Repressive Complex (PRC)2 in cortical development provides a compelling framework for my hypothesis towards the existence of – potentially region- or cell type-specific – tissue-wide control mechanisms keeping individual mutant stem cells in check when existing in a non-mutant environment.
Briefly, I have discovered that PRC2 is not cell-autonomously required for faithful neurogenesis, but rather orchestrates neuron production on the global tissue level in a non-cell-autonomous manner (see Figure below, top level). In other words, I have shown that global tissue effects mediated by a PRC2-sufficient environment provide protective mechanisms to mutant stem cells and instruct normal neurogenic behavior in individual mutant cells. However, at later stages of cortical development, namely astrogliogenesis, single mutant cells do require cell-autonomous PRC2 function for proper astrocyte progenitor proliferation and astrocyte maturation (see Figure below, bottom panel).
The established experimental assays from my postdoctoral work thus provide the profound stepping stones for evaluating the instructive cues mediated by the cellular environment for other essential genes/cellular processes in brain development.
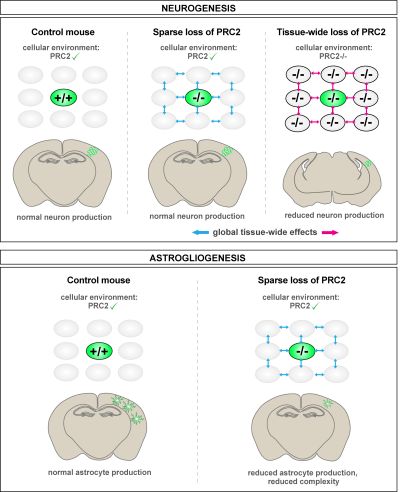
Distinct and sequential PRC2 functions in cortical stem cell lineage progression. Top panel: PRC2 does not cell-autonomously govern excitatory projection neuron production, but is required on the global tissue level. As a result, sparse PRC2-/- stem cells produce a normal amount of neurons, while tissue-wide loss of PRC2 results in reduced neuron production and microcephaly. Bottom panel: PRC2 cell-autonomously controls cortical astrocyte production and astrocyte maturation. Individual PRC2-/- astrocyte progenitors undergo less rounds of proliferation. Furthermore, PRC2-/- astrocytes show reduced morphological complexity.